How to store and use PCR primers
This blog post is the last of 4-part series on PCR primers. Elsewhere on our blog, you can find posts on understanding PCR primers, designing quality primers, and ordering primers.
So, you’ve just ordered new primers. How do you prepare them for use in a PCR experiment?
Primers (or “oligos” for “oligonucleotides”) are typically mailed to you as a dehydrated pellet—though note that many suppliers now offer pre-mixed primers already diluted to your desired stock concentration. If you ordered pre-mixed primers (which may be branded as “RxnReady” if you ordered from IDT), congratulations: you do not need to make a stock solution, and can advance directly to step 2 below. If your primers arrived dehydrated, your first step should be rehydrating your primers and freezing them for long-term storage.
Step 1: Make a stock solution for long-term storage
To rehydrate your primers, you add nuclease-free water or TE buffer (10 mM Tris pH 8.0, 1 mM EDTA). Oligos are highly soluble and will dissolve almost instantly. While there is no rule for the concentration at which you should store your primers, we typically make a 100 μM stock concentration in TE buffer. This is somewhere around 100-1,000 times the concentration you will use in a typical PCR. Then, as you need to use the primers, you can make a working stock, typically in nuclease-free water (see step 2). This will help protect your stock primer from contamination and repeated freeze-thaws.
The amount of primer you receive is usually reported to you in nanomoles, so a simple trick to make a 100 μM solution is to take the reported nanomoles of primer, multiply it by ten, and then add that many microliters of water. For example, if you receive 20 nmoles of primer, adding 200 μl of nuclease-free water will give you a final concentration of 100 μM. Primers stored in nuclease-free water or TE are typically quite stable and can be stored in the freezer for years.
Step 2: Make a working stock for PCR experiments
When you are ready to set up your reactions for PCR, dilute some of your stock in nuclease-free water to create a working stock. What concentration should you use for your working stock? Final primer concentrations in a PCR are typically between 100 nM and 1 μM. But remember, once you account for the addition of your other PCR reagents—polymerase, dNTPs, and buffer, likely in the form of an all-in-one Master Mix—your final primer concentration will be more dilute than your working stock.
Depending on how you plan to use your primers, you may wish to keep one working stock that contains both primers or two separate working stocks, one for your forward and one for your reverse primer. If you plan on always using these two primers as a pair and not ever trying them with other primers, you can make a single working stock containing the correct concentration of both primers. If you plan to mix and match primer pairs, you will be better off keeping separate working stocks.
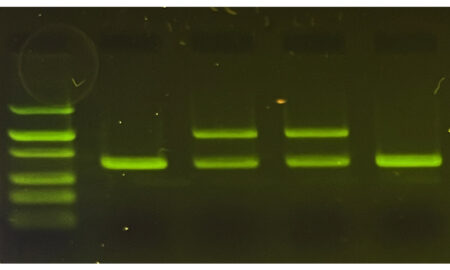
What precise concentration is used is usually decided by empirically testing primers and seeing what works best. A weak PCR product may suggest trying a higher primer concentration. Strong primer dimer or off-target bands may suggest using a lower primer concentration.
Step 3: Storing your PCR primers
Now that you have primer stock and working solutions, what are the ideal storage conditions to promote long term stability? For long-term primer storage, temperature is the most important factor to consider. Whether they are dissolved in nuclease-free water or TE buffer, we recommend that you store primer stock solutions frozen at –20 °C. For everyday use, working primer solutions are stable at 4 °C for many weeks, or even months.
Contributed by miniPCR bio curriculum director Bruce Bryan